Motion Preserving Cervical Spine Surgery

Motion preserving cervical spine surgery compared to spinal fusion
The current standard of care for the surgical treatment of cervical disc disease is fusion, which is known as anterior cervical discectomy and fusion.
In both an anterior cervical discectomy and fusion procedure, and a motion preserving procedure, the unhealthy disc is removed and the height at that level is restored to relieve pressure on the nerves and/or spinal cord.
In an anterior cervical discectomy and fusion, after the unhealthy disc is removed, it is replaced with a bone graft or synthetic spacer, and a cervical plate with screws is used for stabilization. The goal of this procedure is to permanently fuse two or more vertebrae together so they cannot move except as a single unit. This may alleviate pain and other symptoms but has potential disadvantages, including loss of motion and flexibility.
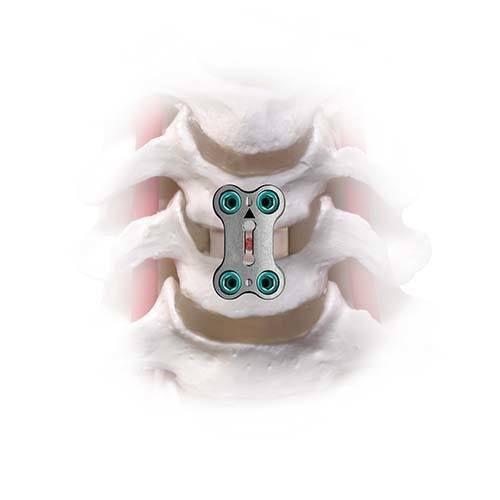
Anterior cervical discectomy and fusion
The SECURE™-C Cervical Artificial Disc has been developed to provide pain relief while allowing motion of the cervical spine.
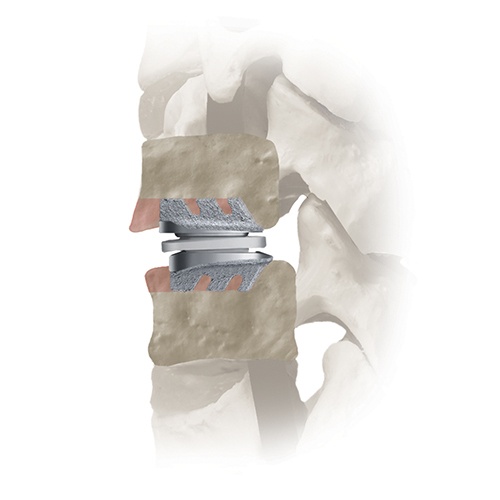
SECURE-C™
SECURE-C™ Cervical Artificial Disc
The SECURE™-C Cervical Artificial Disc consists of two metallic endplates (cobalt chromium molybdenum alloy, CoCrMo) and a polyethylene inner core. The materials used in the device are commonly used in orthopedic implants.
The two endplates are secured to the top and bottom surfaces of the involved vertebrae and the core fits between them. The implanted device is designed to allow motion at the treated level as the plastic core moves against the metallic endplates.

SECURE-C™ endplates and inner core
SECURE™-C’s design is intended to allow the neck to move in flexion/extension, lateral bending and axial rotation.
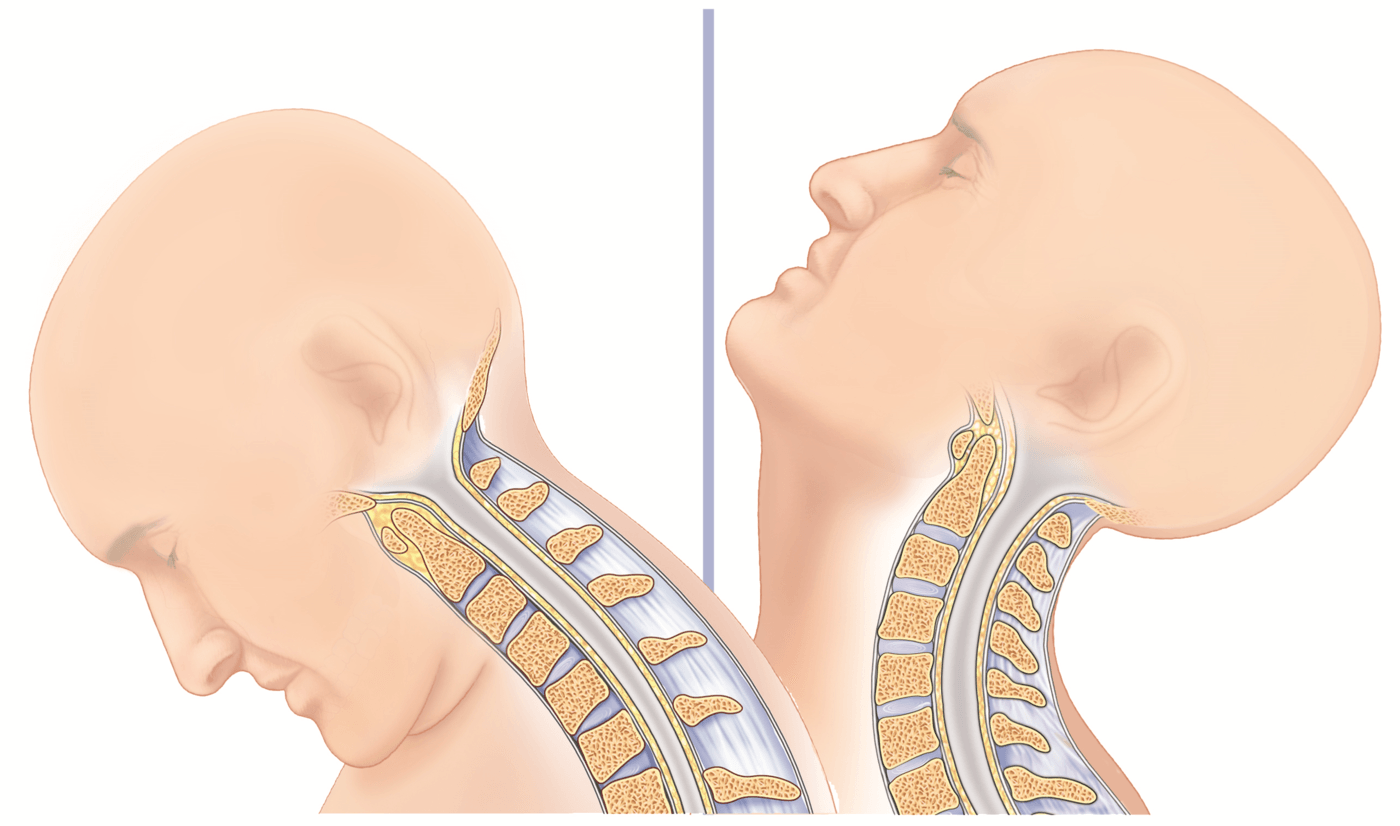
Flexion and extension of the neck
SECURE™-C is intended to treat a disc in the cervical spine between the C3 and C7 vertebral bodies. The device is provided in different sizes to fit different patients.
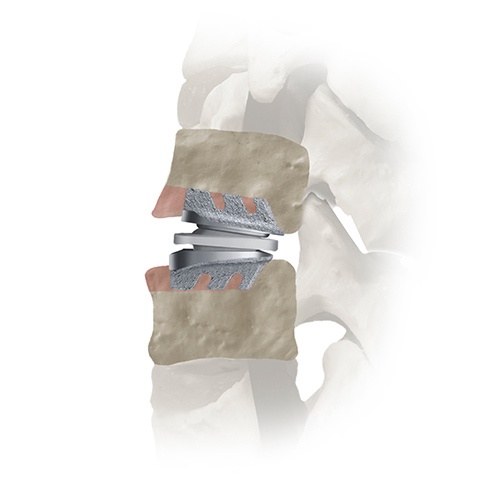
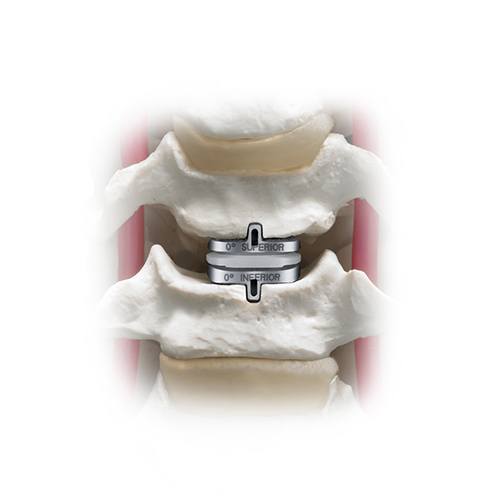
Placement of SECURE-C™ in the vertebral body
Who should receive the SECURE-C™ Cervical Artificial Disc?
The SECURE-C™ Cervical Artificial Disc may be used to treat patients who meet the following requirements:
- 21 to 60 years old
- One diseased disc (C3-C7)
- Arm pain and/or neurological symptoms such as weakness or numbness with or without neck pain for at least six weeks that has not responded to non-surgical care such as medication and physical therapy
- Specific findings on imaging studies such as X-ray, CT, or MRI
In addition, in order to receive this device, you must be old enough so that your bones are mature and no longer growing.
Disclaimer:
The material on this website is intended to be an educational resource only and is not meant to be a warranty or to replace a conversation between a patient and their physician or member of their health care team. Please consult a physician for a complete list of indications, contraindications, precautions, warnings, clinical results and other important medical information that pertains to this procedure. The decision to receive medical treatment is individualized to the patient and the patient’s symptoms. The information presented on this site may not apply to your condition, treatment or its outcome, as surgical techniques vary and complications can occur. It is important to discuss the viability of any surgical procedure with a physician to decide the right treatment option.
